Surgical Face Mask
Product Lifecycle
Quality Management
Testing, Inspection, Audit and Certification for Face Masks
Face Mask Product Lifecycle Quality Management
Download Service SheetBUSINESS CHALLENGE
In order to satisfy the expanding demand for surgical masks worldwide, manufacturers all over the world are changing their production lines. How confident are you, as a buyer or seller, that your procedure and product controls will help you achieve brand and regulatory requirements during these difficult times?
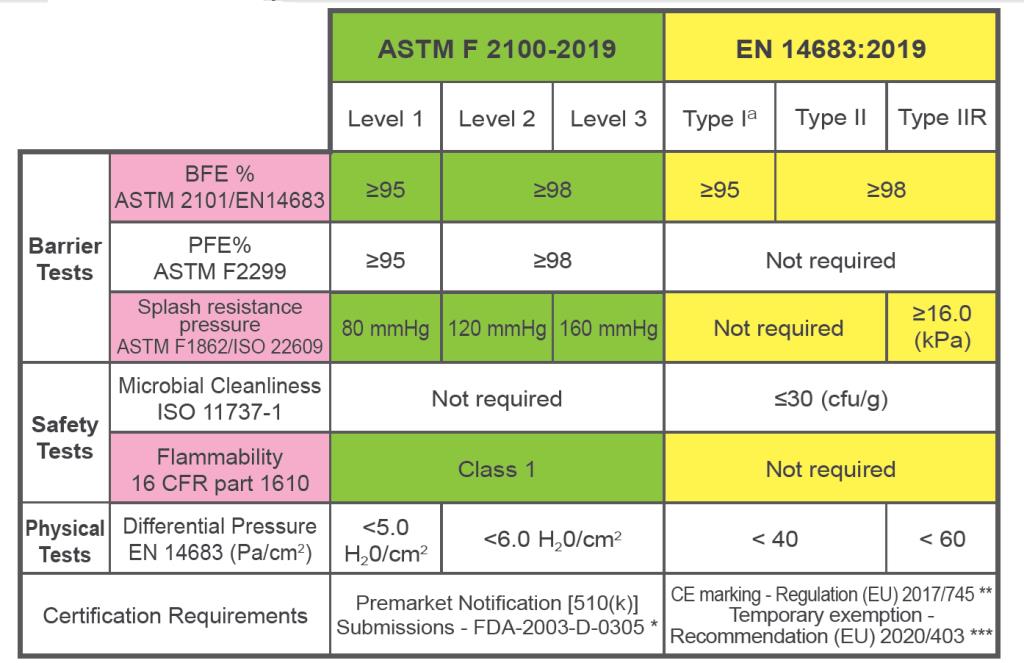
The table below provides specifics on the testing and certification standards for the USA (ASTM) and Europe (EN) for two of the main markets:
* Premarket Notification [510(k)] Submissions - FDA-2003-D-0305
** CE marking - Regulation (EU) 2017/745
*** Temporary CE marking exemption - Recommendation (EU) 2020/403
In addition, we have JIS T9001 for Japan market, TCVN 8389-1/2/3 for domestic Vietnam, etc.
TEMPORARY CE MARKING EXEMPTION
RECOMMENDATION (EU) 2020/403 was published on March 13, 2020, to address the COVID-19 threat and the surgical face mask scarcity.
When surgical face masks without the CE mark are slated to enter the EU market, the relevant market surveillance authorities will inspect the products and, if they are found to meet the minimum health and safety standards, will take the necessary steps to permit their sale on the Union market for a brief period of time, or while the conformity assessment process with the notified body is underway.
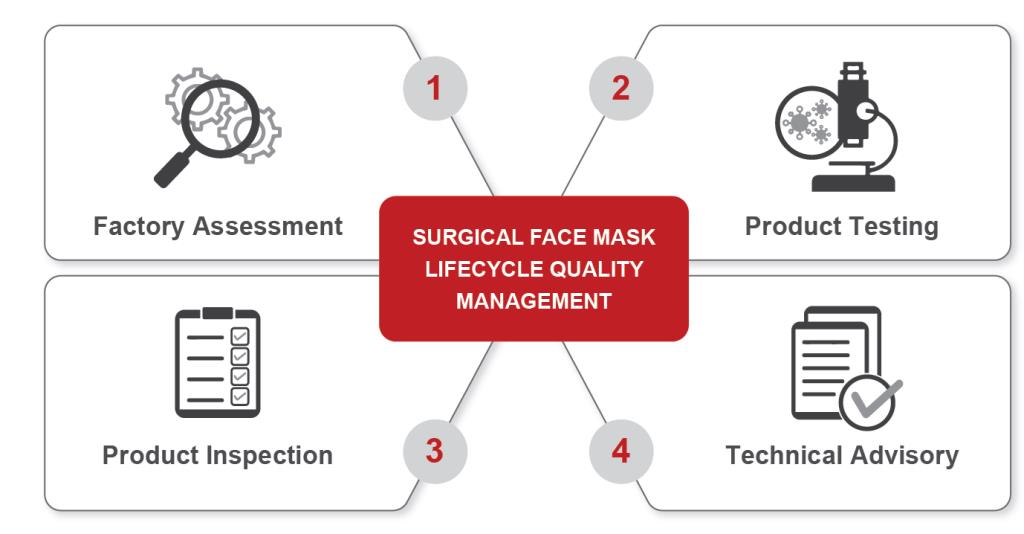
BUREAU VERITAS SOLUTIONS
Factory Assessment: Supports the evaluation of factory compliance with the particular arrangements made to handle the COVID-19 outbreak. Sterilization facilities, disinfection spaces, clean rooms, appropriate protective clothing, storage, and other needs are specific to surgical face masks.
Product Testing: The Microbiological Hub in Sri Lanka conducts Bacterial Filtration Efficiency (BFE) and related tests on surgical face masks in accordance with EN 14683 and ASTM F2101, its counterpart committee requirements in the U.S. (ASTM F2101).
Product Inspection: Bureau Veritas’s inspectors had been trained in major face mask production countries worldwide. The inspection methodology covers quantity, labeling, specification review, and a number of on-site tests, such as:
- product dimension measurement;
- nose clip reliability;
- tensile test to the mask connection points;
- claimed function;
- actual wear test;
- and more.
Technical Advisory:
- Level 1: Document Review: Checking that the documentation, which includes the pertinent test requirements, third-party lab accreditation, vendor information, and overall rating, is adequate;
- Level 2: Access with CE designation. Our experts can assist you in receiving official CE marking from a designated Notified Body or exemption acceptance from a surveillance body.
FAQ - Face Mask Compliance & Quality Control
Download FAQ